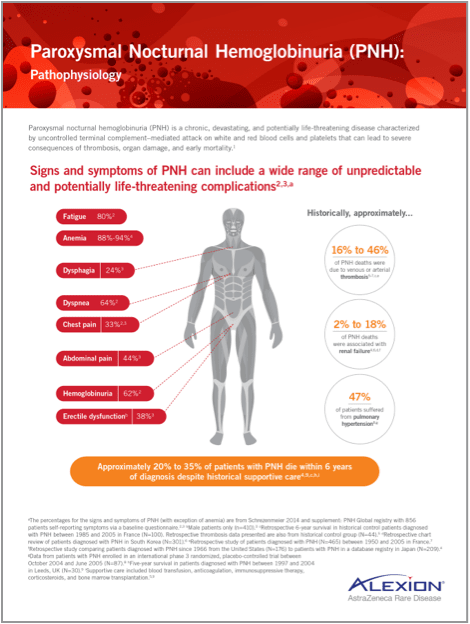
Signs and symptoms of PNH
Signs and symptoms of PNH can include a wide range of unpredictable and potentially life-threatening complications1,2,a

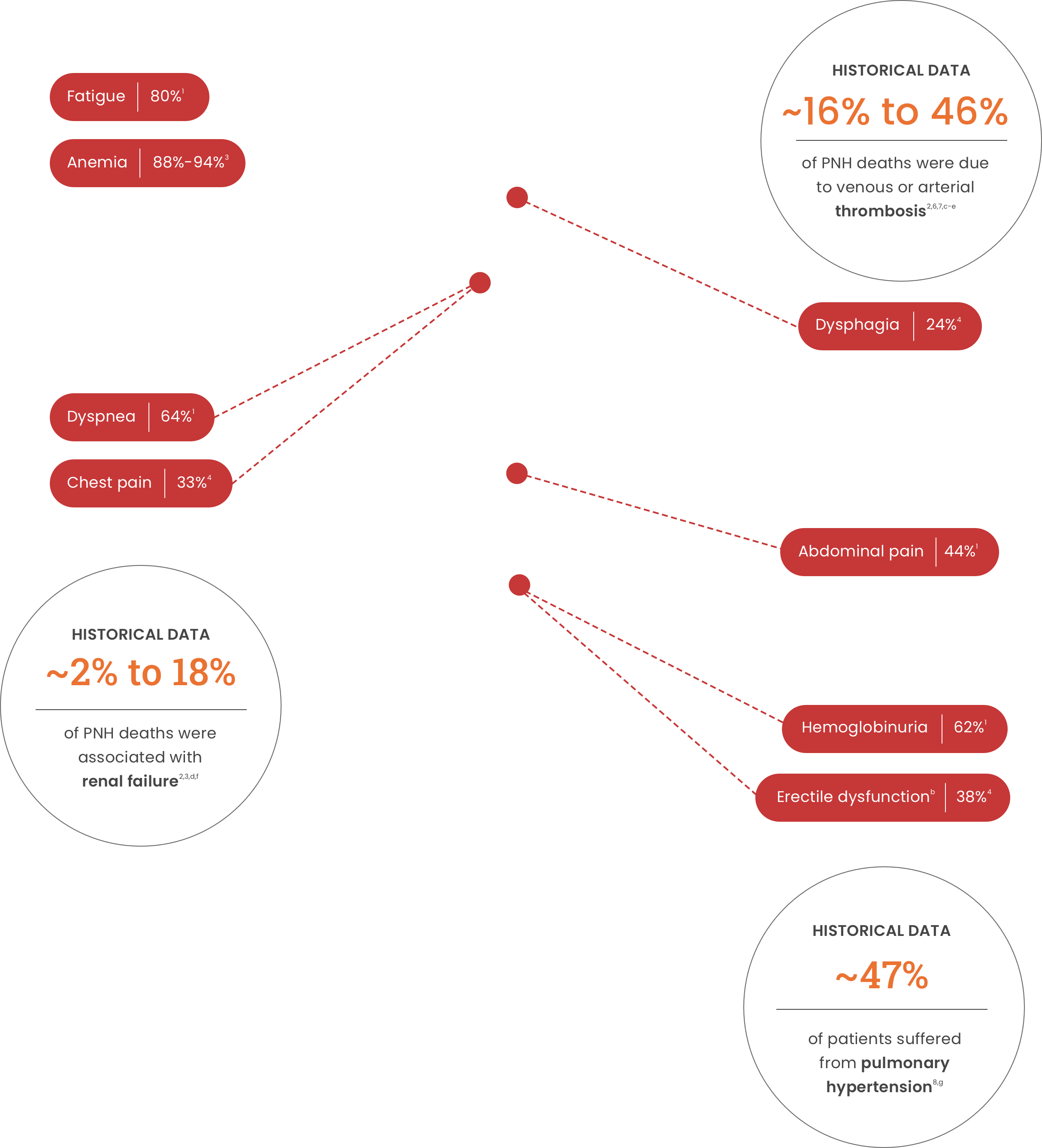
Anemia88%-94%3 Dysphagia24%4 Dyspnea64%1
Chest pain33%4 Abdominal pain44%1 Hemoglobinuria62%1
Erectile dysfunctionb38%4
Historical data
of PNH deaths were due to venous or arterial thrombosis2,6,7,c-e
of PNH deaths were associated with renal failure2,3,d,f
of patients suffered from pulmonary hypertension8,g
aThe percentages for the signs and symptoms of PNH (with exception of anemia) are from Schrezenmeier 2014 and supplement: PNH Global registry with 856 patients self-reporting symptoms via a baseline questionnaire.1,4
bMale patients only (n=410).4
cRetrospective 6-year survival in historical control patients diagnosed with PNH between 1985 and 2005 in France (N=100). Retrospective thrombosis data presented are also from historical control group (N=44).6
dRetrospective chart review of patients diagnosed with PNH in South Korea (N=301).2
eRetrospective study of patients diagnosed with PNH (N=465) between 1950 and 2005 in France.7
fRetrospective study comparing patients diagnosed with PNH since 1966 from the United States (N=176) to patients with PNH in a database registry in Japan (N=209).3
gData from patients with PNH enrolled in an international phase 3 randomized, placebo-controlled trial between October 2004 and June 2005 (N=87).8
hFive-year survival in patients diagnosed with PNH between 1997 and 2004 in Leeds, UK (N=30).9
iHistorical supportive care included blood transfusion, anticoagulation, immunosuppressive therapy, corticosteroids, and bone marrow transplantation.6,9
- Schrezenmeier H, et al. Haematologica. 2014;99(5):922-929.
- Jang JH, et al. J Korean Med Sci. 2016;31(2):214-221.
- Nishimura JI, et al. Medicine (Baltimore). 2004;83(3):193-207.
- Schrezenmeier H, et al. Haematologica. 2014;99(5)(suppl):S1-S6.
- Schrezenmeier H, et al. Ann Hematol. 2020;99(7):1505-1514.
- Loschi M, et al. Am J Hematol. 2016;91(4):366-370.
- de Latour RP, et al. Blood. 2008;112(8):3099-3106.
- Hill A, et al. Br J Haematol. 2010;149(3):414-425.
- Kelly RJ, et al. Blood. 2011;117(25):6786-6792.