Absence of GPI anchors
In patients with PNH, an acquired mutation in the PIG-A gene prevents the production of GPI anchors and results in the lack, or reduced expression, of GPI-anchored complement regulatory proteins, leading to dysregulation of the complement system.1,2

Normal red blood cell
Hematopoietic stem cells in bone marrow
Normal hematopoietic stem cells
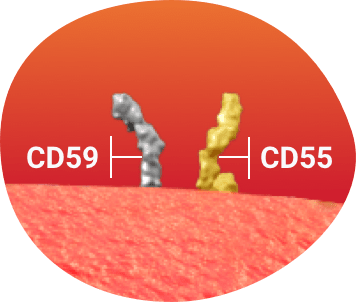
GPI-anchored complement regulatory proteins
In healthy red blood cells, GPI-anchored complement regulatory proteins (CD55 and CD59)a defend against complement–mediated hemolysis.1
aComplement-inhibiting proteins CD55 (decay-accelerating factor) and CD59 (membrane inhibitor of reactive lysis) defend red blood cells against complement–mediated lysis by regulating the formation and stability of the C3 convertase and blocking the assembly of the membrane attack complex, respectively.1

PNH red blood cell
Hematopoietic stem cells with acquired mutation in PIG-A gene
No GPI-anchored complement regulatory proteins
In PNH, an acquired PIG-A mutation can lead to the complete or partial absence of GPI-anchored complement regulatory proteins.1
Red blood cells lacking surface expression of GPI-anchored complement regulatory proteins have increased sensitivity to complement attack.1
White blood cells and platelets without the GPI-anchored complement regulatory proteins are activated by complement attack.1
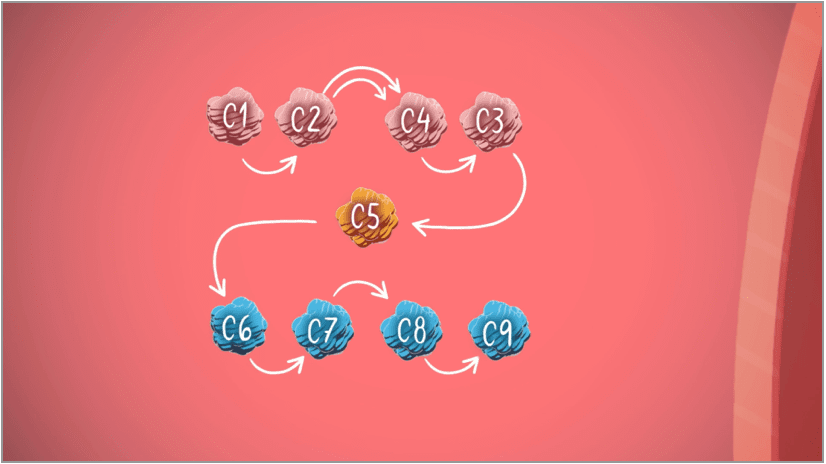
The complement cascade
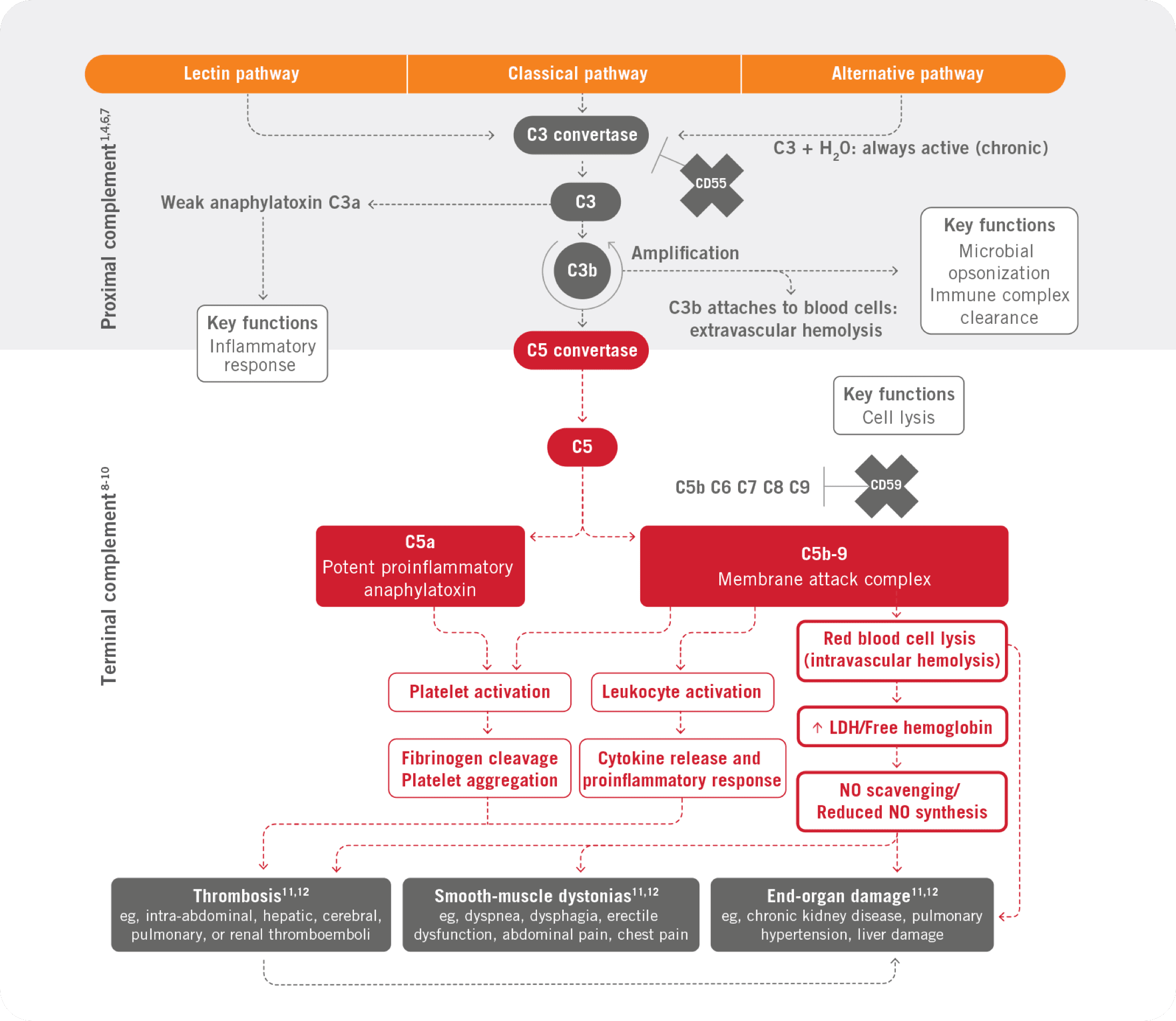
Part of the body's own immune system, called the complement system, is made of 2 parts—the proximal part and the terminal part.4
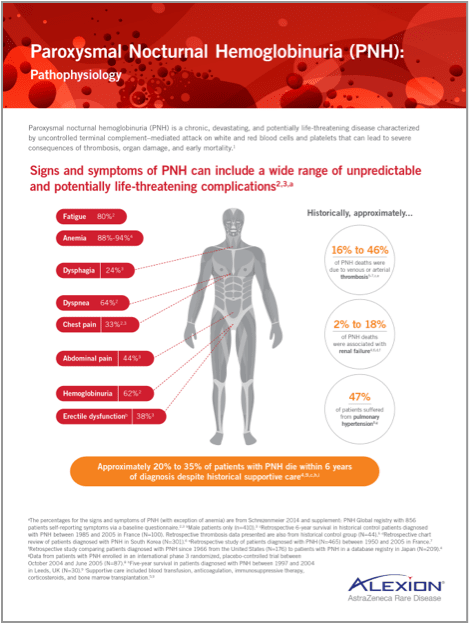
PNH is characterized by terminal complement–mediated attack on red blood cells, white blood cells, and platelets, leading to severe consequences of thrombosis, organ damage, and early mortality5
See full complement cascade diagram
C3

Proximal complement (C3)
Deficiency of the C3 opsonic activities of proximal complement may lead to increased susceptibility to bacterial infection13
C3 convertase activity is an important defense against bacterial infections.13
- Streptococcus pneumoniae
- Staphylococcus aureus
- Escherichia coli
- Haemophilus influenzae
- Neisseria meningitidis
- Streptococcus agalactiae
- Kingella kingae
- Stenotrophomonas maltophilia
- Enterococcal species
- Certain fungal organisms
C5
Terminal complement (C5)
Deficiency in components of terminal complement (eg, C5, C6, C7, C8, or C9) may impact the formation of the membrane attack complex, such as C5b-9, and its ability to lyse certain bacteria15
Deficiencies in terminal complement predispose the patient to infection with meningococcal and disseminated gonococcal infections.15
- Meningococcal infections
- Disseminated gonococcal infections
Consequences of clonal expansion of PIG-A mutant HSCs
In PNH, the key consequences of clonal expansion of PIG-A mutant HSCs are intravascular hemolysis and thrombosis9
Terminal complement–mediated intravascular hemolysis
Terminal complement–mediated intravascular hemolysis is the destruction of red blood cells within the blood vessels as a result of defective red blood cells (as in PNH), complement activation, drugs, or infection17-19
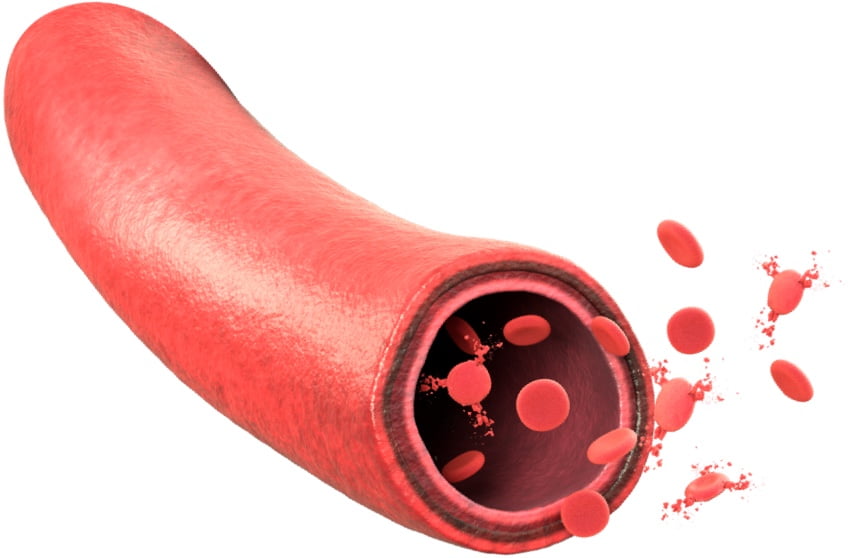
There are limited mechanisms to process the cellular debris resulting from intravascular hemolysis.9,18
When contents of red blood cells spill out into the vessels, it causes multiple pathological consequences.9,18
Intravascular hemolysis can result in marked increase in circulating free hemoglobin as well as enzymes such as LDH.9,18
Patients can often manifest acute, significant anemia in the setting of intravascular hemolysis with evidence of end-organ damage, particularly within the renal system, attributable to terminal complement fixation.9,18
In a small subset of patients with PNH, proximal complement–mediated extravascular hemolysis occurs, leading to symptoms such as residual anemia.9,20
- Parker CJ. Hematology Am Soc Hematol Educ Program. 2016;2016(1):208-216.
- Brodsky RA. Hematology: Basic Principles and Practice. 4th ed. Churchill Livingstone; 2005:419-427.
- Borowitz MJ, et al. Cytometry B Clin Cytom. 2010;78(4):211-230.
- Murphy K, Janeway C. Janeway’s Immunobiology. 8th ed. Garland Science, Taylor & Francis Group LLC; 2012:37-71.
- Sharma VR. Clin Adv Hematol Oncol. 2013;11(9)(suppl 13):2-8.
- Luzzatto L, et al. Br J Haematol. 2011;153(6):709-720.
- Kelly R, et al. Ther Clin Risk Manag. 2009;5:911-921.
- Hill A, et al. Blood. 2013;121(25):4985-4996.
- Hill A, et al. Nat Rev Dis Primers. 2017;3:17028.
- Brodsky RA. Blood. 2014;124(18):2804-2811.
- Bessler M, Hiken J. Hematology Am Soc Hematol Educ Program. 2008;2008(1):104-110.
- Rother RP, et al. JAMA. 2005;293(13):1653-1662.
- Walport MJ. N Engl J Med. 2001;344(14):1058-1066.
- Figueroa JE, Densen P. Clin Microbiol Rev. 1991;4(3):359-395.
- Ram S, et al. Clin Microbiol Rev. 2010;23(4):740-780.
- El Sissy C, et al. Front Immunol. 2019;10:1936.
- Ramaiah L, et al. Haschek and Rousseaux’s Handbook of Toxicologic Pathology. 3rd ed. Elsevier; 2013:1863-1933.
- Siddon AJ, Tormey CA. Adv Clin Chem. 2019;89:215-258.
- Drug-induced immune hemolytic anemia. MedlinePlus. Published January 19, 2021. Updated April 1, 2021. Accessed April 7, 2022. https://medlineplus.gov/ency/article/000578.htm
- Risitano AM, et al. Mini Rev Med Chem. 2011;11(6):528-535.